After surgery I was offered a clinical trial of chemotherapy, which I decided not to enter. A year later I had advancing disease and at that point accepted a clinical trial, which gave me remission. Both were EORTC studies.

WHAT IS CLINICAL RESEARCH?
Scientists and doctors are constantly developing more effective and less toxic treatments to improve patient survival and quality of life. Clinical trials are a form of research that confirms the safety and effectiveness of these new and promising treatments. Many cancer treatments used today are a result of past clinical trials.
WHAT ARE THE OBJECTIVES OF CLINICAL RESEARCH?
In cancer research, clinical trials focus on evaluating new drugs or optimising different therapeutic approaches including surgery, radiation therapy and combination of drugs already on the market.
HOW DO CLINICAL TRIALS EVOLVE?
Oncologists treat their patients with verified, standard treatments and therapies. Standard cancer treatments serve as the base from which new and potentially better treatments evolve. New treatments are tested in clinical trials for effectiveness, tolerance, and toxicity compared to existing treatments.
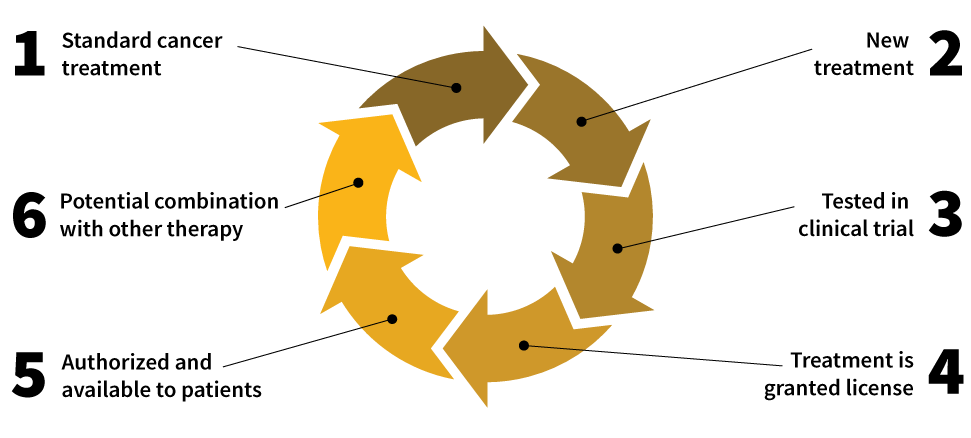
If this new treatment is well tolerated and effective in a large number of patients in the clinical trial, the relevant authorities authorize and grant the treatment a license. Then, the treatment is available to patients and for oncologists to use in combination with other treatments, including surgery or radiation therapy.
WHY PARTICIPATE IN A CLINICAL TRIAL?
Patients take part in clinical trials for many reasons. They are among the first to receive new treatments before they are widely available. However, even though it is a highly screened and monitored environment, it is difficult to judge how the patient will react to the treatment. Clinical trials allow for a safe environment to evaluate the risks and benefits of a new treatment during and after a trial.
Patients also become part of a unique network of clinical trials carried out in Europe and sometimes in cooperation with the USA. Through this network, doctors and researchers are able to pool and share their experience in design and monitoring of clinical trials but most importantly, their knowledge on cancer treatment. By participating in a clinical trial, patients contribute to this important network.
HOW CAN I JOIN AN EORTC CLINICAL TRIAL?
If you would like to know more about joining an EORTC trial, please visit the EORTC Website where all open trials are listed by tumour types. You may also find all EORTC trials at https://clinicaltrials.gov/



