SOFT TISSUE & BONE SARCOMA GROUP
EORTC 1809 – STRASS 2/ STREXIT 2 – A randomized phase III study of neoadjuvant chemotherapy followed by surgery versus surgery alone for patients with High Risk RetroPeritoneal Sarcoma (RPS)
Retroperitoneal sarcoma is a very rare tumour, occurring in 0.5 to 1.0 per 100,000 of the population. Diagnosis and treatment are challenging. The standard of care consists currently of surgery. Overall patient survival at 5 years following surgery is between 60 and 70%.
The EORTC 1809 STRASS 2 study is the first official trial looking into potential benefits of chemotherapy before surgery to improve disease control and survival in patients burdened by this rare cancer. June 2023 signals the start of a new observational arm called STREXIT 2, attached to the ongoing clinical trial in order to capture real world data from patients who are not eligible to participate in STRASS 2.
The aim is to compare the clinical outcomes between STRASS 2 and STREXIT 2 and to explore the possible combination of STRASS 2 and matched STREXIT 2 patients. This will strengthen the results obtained from the randomised clinical trial data and increase the power of subgroup analyses.
The early incorporation of real-world data (STREXIT 2) into the trial (STRASS 2) will enrich the study population and provide a pragmatic approach to addressing crucial causal questions within a rare cancer population.
Home - Soft Tissue & Bone Sarcoma Group
SOFT TISSUE & BONE SARCOMA GROUP
EORTC 1809 – STRASS 2/ STREXIT 2 – A randomized phase III study of neoadjuvant chemotherapy followed by surgery versus surgery alone for patients with High Risk RetroPeritoneal Sarcoma (RPS)
Retroperitoneal sarcoma is a very rare tumour, occurring in 0.5 to 1.0 per 100,000 of the population. Diagnosis and treatment are challenging. The standard of care consists currently of surgery. Overall patient survival at 5 years following surgery is between 60 and 70%.
The EORTC 1809 STRASS 2 study is the first official trial looking into potential benefits of chemotherapy before surgery to improve disease control and survival in patients burdened by this rare cancer. June 2023 signals the start of a new observational arm called STREXIT 2, attached to the ongoing clinical trial in order to capture real world data from patients who are not eligible to participate in STRASS 2.
The aim is to compare the clinical outcomes between STRASS 2 and STREXIT 2 and to explore the possible combination of STRASS 2 and matched STREXIT 2 patients. This will strengthen the results obtained from the randomised clinical trial data and increase the power of subgroup analyses.
The early incorporation of real-world data (STREXIT 2) into the trial (STRASS 2) will enrich the study population and provide a pragmatic approach to addressing crucial causal questions within a rare cancer population.
Clinical Trial
Phase III
Cancer type
Sarcoma
Set up

EORTC & Intergroups
Status

Recruiting
Clinical trial:
Phase III
Cancer type:

Sarcoma
Set up:

EORTC & Intergroups
Status:

Recruiting
KEY STUDY MILESTONES
-
EORTC Board endorsement
EORTC Board endorsement
- 04/11/2019
-
Regulatory submissions (start)
Regulatory submissions (start)
- 29/09/2020
-
First Site Active (FSA)
First Site Active (FSA)
- 20/01/2021
-
STREXIT 2 Activation
STREXIT 2 Activation
- March 2026
-
End of STREXIT 2
End of STREXIT 2
- Estimated mid 2028
-
Final Analysis Report (FAR)
Final Analysis Report (FAR)
- June 2030
-
Publication of results
Publication of results
- 21/03/2018
-
Protocol Release
Protocol Release
- 28/05/2020
-
Database set-up
Database set-up
- 06/10/2020
-
First Patient In (FPI)
First Patient In (FPI)
- November 2023
-
Last Patient In (LPI) – 250 patients
Last Patient In (LPI) – 250 patients
- May 2028
-
STRASS 2 end of accrual
STRASS 2 end of accrual
- December 2029
-
Long-term patient Follow-up / End of Project
Long-term patient Follow-up / End of Project
- June 2030
KEY STUDY MILESTONES
-
EORTC Board endorsement
EORTC Board endorsement
- 04/11/2019
-
Regulatory submissions (start)
Regulatory submissions (start)
- 29/09/2020
-
First Site Active (FSA)
First Site Active (FSA)
- 20/01/2021
-
STREXIT 2 Activation
STREXIT 2 Activation
- March 2026
-
End of STREXIT 2
End of STREXIT 2
- Estimated mid 2028
-
Final Analysis Report (FAR)
Final Analysis Report (FAR)
- June 2030
-
Publication of results
Publication of results
- 21/03/2018
-
Protocol Release
Protocol Release
- 28/05/2020
-
Database set-up
Database set-up
- 06/10/2020
-
First Patient In (FPI)
First Patient In (FPI)
- November 2023
-
Last Patient In (LPI) – 250 patients
Last Patient In (LPI) – 250 patients
- May 2028
-
STRASS 2 end of accrual
STRASS 2 end of accrual
- December 2029
-
Long-term patient Follow-up / End of Project
Long-term patient Follow-up / End of Project
- June 2030
A word from…
Study Coordinators
& Partners
Patients
A word from…
Study Co-ordinators & Partners
Patients
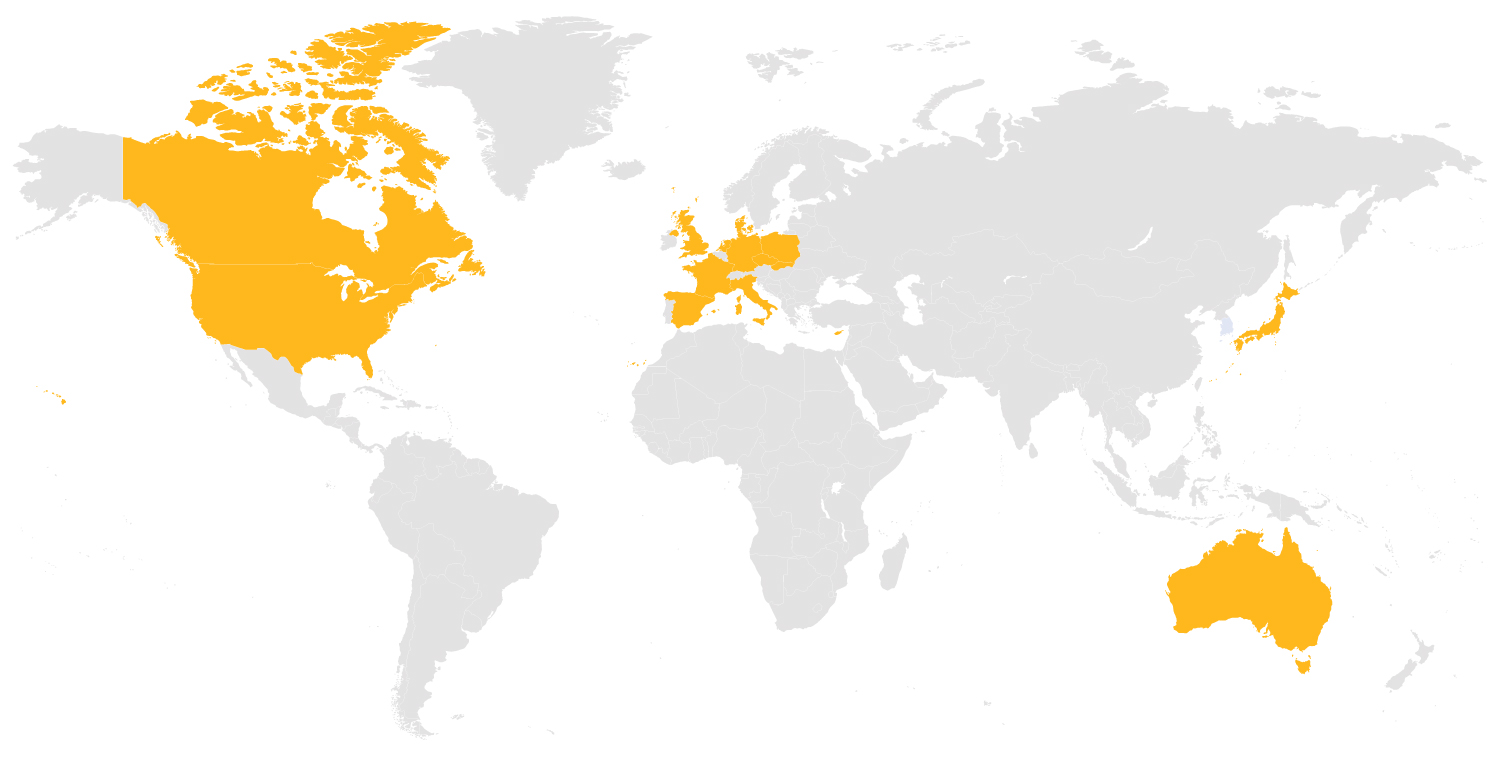
STUDY STATUS
To view the Study Status map, please flip your phone horizontally:
Video Series
STRASS2/STREXIT 2 – Introduction
Dr Winan van Houdt, introduces STREXIT2